Accutane: Is it Worth the Risk?
By
Andrea Long
21 May 2002
With the drug industry on a rise, more and more drugs appear on the marketplace everyday. We have all seen the ads on television, with pictures of happy people canoeing or bike riding and an upbeat voice extolling the drug‚s virtues. Few people pay attention as the announcer continues in the same cheery voice to list the drug‚s possible side effects. Still fewer people fully examine the side effects of some of the older drugs, even as previously unknown complications develop. One such drug is Accutane, and the side effects of this particular drug are damaging, to say the least.
Accutane was first
introduced to the public after its FDA approval in 1982. Accutane comes in a
gelatin capsule of 10 mg, 20 mg, or 40 mg size, and its active ingredient is
isotretinoin. The inactive ingredients include beeswax,
butylated hydroxyanisole, edetate disodium, hydrogenated soybean oil flakes,
hydrogenated vegetable oil, and soybean oil. Gelatin capsules contain glycerin and
parabens (methyl and propyl), with the following dye systems: 10 mg ų iron
oxide (red) and titanium dioxide; 20 mg ų FD&C Red No. 3, FD&C Blue No.
1, and titanium dioxide; 40 mg ų FD&C Yellow No. 6, D&C Yellow No. 10,
and titanium dioxide. Isotretinoin also displays a high lipophilicity, which
makes ingestion with a high fat meal a distinct enhancement in its
effects. (1)
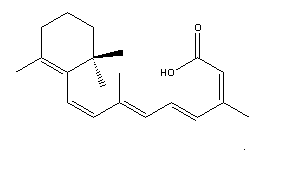
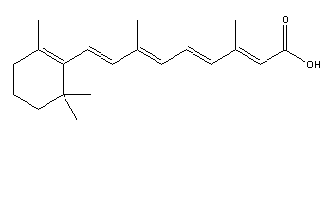
The biologically active form of isotretinoin is 13-cis-retinoic acid (Figure 1). This is a synthetic stereoisomer of all-trans retinoic acid, also known as tretinoin (Figure 2), and the two isomers display reversible interconversion. (2) After ingestion of the capsules, isotretinoin binds to proteins in plasma, particularly albumin. Oxidative metabolism occurs in the liver cytochrome P450 system, and produces 4-oxo-isotretinoin and geometrical isomer 4-oxo-tretinoin. (3) The oxidation process is irreversible.
Figure 1: Isotretinoin (C20H28O2 ) Figure 2: Retinoic acid (C20H28O2 )
Courtesy of
chemfnder (4)
Although Accutane has been used for more than twenty years, and researched for an even longer period of time, its exact mechanism remains unknown. However, one of the results is an attenuation of the sebaceous glands and a resultant decrease in sebum secretion. The decrease is only temporary, though. (2)
Its primary use is in treating severe nodular or cystic acne. These types of acne involve inflammation and lesions that extend deeply into the skin, and cause pain and possible scarring. (5) They are also notably resistant to convential types of treatment, such as topical creams andalcoholic solutions. Thus, the acne suffering population received this „miracleš drug with delight. More and more doctors started dispensing the drug that their patients so desparately wanted, without fully conveying the wide range of hazardous side effects that Accutane can have.
One of the most basic side effects of Accutane stems from its relationship to Vitamin A. As a retinoid, Accutane has some of the same actions in the body as Vitamin A, and thus if any more of the vitamin is introduced, it may accentuate the effects even more. This reaction is near near universal and causes skin rashes, conjunctivitis, stomatitis, and dry skin. (3) Increased reaction to sunlight can also occur.
Another, very serious, side effect is Accutane‚s teratogenicity. Accutane has been classified as a Category X teratogen by the FDA. The risk of spontaneous abortion and premature birth is high, but that is not the worst danger. The chance for fetal abnormality, both external and internal, and also mental retardation, is greatly increased by even a short exposure to Accutane. Documented cases include abnormalities of skull, eye, CNS, cardiovascular system, thyroid and parathyroid glands, and many others. Basically, Accutane can damage just about any part of the child‚s anatomy. (2) The disastrous mutations are possibly due to the action of isotretinoin in cells. Isotretinoin binds to a cytoplasmic protein called the retinoic acid binding protein (CRABP) and is transported to the nucleus of the cell. Retinoid-dependent receptors known as RAR and/or RXR then bind to the isotretinoin complex and to target DNAs. This results in transcription and subsequent expression of cellular genes involved in cell growth, proliferation, and differentiation. (3) If this reaction were to occur in the cells of a fetus, the normal development would be interfered with and mutations and abnormalities would arise.
More, less rearched side effects, are possible psychiatric consequences. More attention came to bear upon the drug as a result of the death of Representative Bart Stupak‚s teenaged son B.J. in May of 2000. Mr. Stupak and his wife began to research the possible relationship between Accutane and their son‚s suicide and were surprised to find an FDA report warning of a possible linkage. Yet, their doctor did not mention the possibility, nor did the drug information sheet with which they were presented. After discovering all the neglect and mismanagement involved in their son‚s case, the Stupaks went on to organize a campaign to educate all the Accutane users in the nation. (6) In response to pressure from the FDA, Roche Laboratories, Inc., the manufacturer of Accutane, added a new warning to their drug information sheet:
Warnings: Psychiatric
Disorders: Accutane may cause depression, psychosis, and, rarely, suicidal
ideation, suicide attempts, and suicide. Discontinuation of Accutane therapy
may be insufficient; further evaluation may be necessary. No mechanism of
action has been established for these events.
This warning is watered down, however, by a statement in the Drug Information sheet that maintains, „No one knows if Accutane caused these behaviors or if they would have happened even if the person did not take Accutaneš. (1) Most patients tend to ignore or downplay possible side effects, and this statement only makes ignoring the risks that much easier. It also denies responsibility for any actions influenced by the psychiatric side effects. So, although the psychiatric effects are mentioned, the miscommunication and ingnorance which affected the Stupaks‚ decision to treat their son with Accutane has not really been resolved.
Due to the increased attention to Accutane, new laws concerning dispensation have been developed. Roche has formed a new policy in order to minimize some of the ignorance of both patients and doctors alike. The System to Manage Accutane Related Teratogenicity (S.M.A.R.T.) was instituted on April 10, 2002 and created an immediate change in dispensing procedures. Prescribers must go through a qualification process involving reading the manufacturer‚s booklet, going through a checklist, and signing a letter acknowledging understanding of the medicine and responsibility for informing patients of the high risk involved in using Accutane. Once qualified, the doctor is provided with yellow qualification stickers that must be applied to the written prescription. (2)
For male patients, the yellow sticker is automatically applied after he has signed a Patient Information/Consent form. For female patients, however, the qualification is more rigourous. In addition to signing the Patient Information/Consent form, she must have two negative urine or serum pregnancy tests prior to dispensation of the medicine. Pregnancy tests are further required once a month for every month the patient is on Accutane. Also, the patient must commit to using two different forms of birth control, to further minimize the risk of getting pregnant. These types are divided into primary and secondary forms. Primary methods include hysterectomy, partner‚s vasectomy, tubal ligation, birth control pills, and intrauterine devices. Secondary schemes involve the use of condoms, diaphragms, and cervical caps, in conjunction with spermicide. (2)
Under S.M.A.R.T., new laws also affect pharmacists. No more telephoned prescriptions are allowed, as each prescription needs a yellow qualification sticker. Regardless of what the prescriber writes, no prescriptions may be filled for more than a 30 days supply, and no refills are allowed. Also, prescriptions may only be filled within seven days of the date written on the qualification sticker. Any prescription received after the sevan day period is considered to be expired. (2)
Although these new laws go a long way to allievating the ignorance of both doctors and patients where Accutane is concerned, they cannot possibly eliminate it altogether. Even the researchers of isotretinoin are surrounded by ingnorance. They have not discovered the precise mechanism by which isotretinoin acts in the human body, and therefore do not know the precise side effects which the mechanism generates. Instead of admitting their lack of knowledge to the population at large, however, Roche manufacturer continues to produce and market this „miracleš drug as the best way to treat acne. Never mind that suicide and depression may result; pay no attention to the reoccurrence of acne after treatment has stopped. As mentioned earlier, the decrease in sebum production is only temporary. The percentage of Accutane users who have a reoccurrence of their acne after treatment has stopped is high and only rises with each subsequent treatment. The drug companies want you you to ignore this fact and concentrate only on the results obtained during the specific time period of treatment. Yes, lessening the effects of sever acne is a good thing, but one must ask one‚s self if the side effects are worth the results when they last for such a short time. People want the miracle cure, and some are always willing to overlook the bad side in order to achieve their goal. But is the „cureš really worth the other consequences of the drug?
Works Cited
1. FDA/Center for Drug Evaluation and Research (May 2002). <http://www.fda.gov/cder/drug/infopage/accutane/medicationguide.htm> Accessed 9 May 2002.
2. Hoffman-LaRoche, Inc (2001). Accutane Medication Guide. <http://www.rocheusa.com/products/accutane/> Accessed 26 April 2002.
3. CancerSource (2001). <http://www.cancersourcemd.com/drugdb3/detail.cfm?ContentID=19834&trade=1> Accessed 8 May 2002.
4. Chemfinder (2002) <http://www.chemfinder.com/> Accessed 9 May 2002.
5. American Academy of Dermatology (2002). <http://www.skincarephysicians.com/acnenet/acne.html> Accessed 26 April 2002.
6. Stupak, Bart (October 2000). Accutane. United States House of Representatives. <http://www.house.gov/stupak/accutane_stupak_statement.htm> Accessed 9 May 2002.