| Back to First Page (index) |
Taxol, a substance originally isolated from the Pacific yew tree (Taxus brevifolia) more than two decades ago, has recently been approved for the clinical treatment of cancer patients. Clinical trials are being run by the National Cancer Institutes (NCI) in the USA and the drug has been made available for patients in the UK (Nursing Times, 1994).
The first clinical trials with Taxol were carried out in 1983 and by 1988 preliminary results were beginning to show promise in patients with ovarian cancer. In the early studies involving patients with progressive disease, more than 30% experienced tumor shrinkage and at least half of those had a response that lasted longer than a year. The latest results from NCI show that 23% of patients with ovarian cancer who have had at least two previous courses with chemotherapy, respond to Taxol and achieve a tumor shrinkage lasting an average of four months (Nursing Times, 1994).
Hailed as having provided one of the most significant advances in cancer therapy, Taxol exerts its anticancer activity by inhibiting mitosis (Nature, 1994). This exciting new anticancer drug has shown clinical activity against ovarian and breast cancer. In fact, Taxol is the first chemotheraputic agent to have demonstrated a significant effect on the progression of ovarian cancer. The current focus of interest, however, has moved to the development of improved analogs of the drug.
Taxol seems to be the answer to many cancer patients' prayers, but there are "deep rooted" ethical issues involved that makes its production a controversial issue. Ecologically, the problem with Taxol is that it is produced from a resource that is rapidly being depleted. It is extracted from the bark of the Pacific yew tree, a slow growing tree native of the pacific north west. It takes an average of six, one hundred year old trees to treat each patient. The scarcity of Taxol and the ecological impact of harvesting it have prompted extension searches for alternative sources including semisynthesis, cellular structure production and chemical synthesis. The later has occurred for almost two decades, but these attempts have been thwarted by the magnitude of synthetic challenge.
The first semisynthetic version of pacitaxol (Taxol) reached the US market in 1995. This semisynthetic version uses the needles and twigs of the easily grown Taxus baccata, the European yew. With this renewable resource, both oncologists and ecologists have been able to breathe more easily. This does not mean that research and development are over. Drug companies, biotech firms, and academic researchers are still working intensely on taxanes- a family that includes pacitaxel, its close cousin docetaxel, and a host of other relatives (Journal of the National Cancer Institute, 1995).
 |
Taxol’s
first complete synthesis was performed independently by
two groups in February, 1994. One group was led by K.C.
Nicolaou of the Scripps Research Institute, the other by
Robert Holton of Florida State University. The complete
synthesis of taxol has eluded chemists for the prior
twenty years, since its anti-cancer potential was
discovered. The ability to synthesize taxol is of great importance, as chemists will now be able to concoct modified versions of the drug. Natural taxol suffers from poor solubility, which makes it difficult to administer. Nicalaou says, "We might find one [modification] that is less toxic and more effective than [natural] taxol. There are a lot of advances to be made" (Science, 1994). Both methods of synthesis take more than 30 steps. This is not considered commercially viable. Yields are reported to be about 0.05%. The first step after synthesis is to find a more effective, modified product, and then perfect a method of synthesis that contains a workable number of steps—less than 25 according to National Cancer Institute chemist Mathew Suffness (Nature, 1994). |
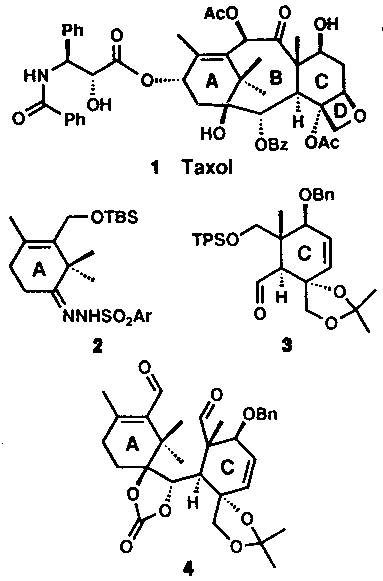
| Taxol
presents a formidable synthetic challenge with its eleven
stereocenters and dense array of functionality. Nicalaou
and colleagues’ synthesis is both convergent—it
employs the fully functionalized A-ring and C-ring
fragments 2 and 3 (see above
figure)—and flexible in that it should allow
the construction of numerous analogs. The first carbon-carbon bond between rings A and C was created through reaction of the carbanion generated from sulphonylhydrazone (2) and aldehyde (3) (Shapiro reaction). Subsequent manipulation of the functional groups provided the dialdehyde (4), and ring B was closed using a McMurry reaction mediated by titanium trichloride and activated zinc. The yield of this step was very low (23%), but the rest of the synthesis, which included oxetane formation, oxygenation of ring A and introduction of the side-chain, proceeded without major problems (Nature, 1994). |
|
Biological Process: How Taxol Works
In book VI of his Gallic Wars Caesar tells us that Catuvolcus, a chieftain of the Eburones, committed suicide by taking an extract of the yew tree. This was a common form of poisoning in ancient times. But it was only in 1964 that the broad-spectrum cytotoxicity of extracts from the Pacific yew was discovered.
A normal cell divides by mitosis—a process of chromosome motions that produce two genetically identical daughter cells. A cancerous cell, or a collection of cancerous cells (a tumor), divides uncontrollably via mitosis. An important mitotic structure is the microtubule. The microtubules create a cytoskeleton within the dividing cell, and allow motion of the chromosomes.
Taxol stops the uncontrolled cell divisions of cancer by forming extremely stable and nonfunctional microtubules. The microtubules are the means of chromosome motion during mitosis (cell division). Mitosis is halted when the stable, nonfunctional microtubules fail to form a normal mitotic apparatus.
Taxol is unique among chemotherapeutic agents as it has a specific binding site on the microtubule polymer. Its ability to polymerize tubulin in the absence of cofactors is also unusual. The presence of taxol reorganizes the cell’s cytoskeleton, blocking cells in the second growth and mitotic phases of the cell cycle (Annals of Oncology, 1994).
Like many other chemotherapies, Taxol also has its side effects. They include hair loss, gastrointestinal disturbances, hypersensitivity reactions, nerve damage and bone marrow depression. The hypersensitivity reactions were initially life-threatening but they are now controlled with a strict regime of premedication. The severity of the neutropenia is now being reduced with a bone marrow stimulating medication called GCSF.
Download the (free) Chime Plug-In
This interactive tutorial requires the plug-in, Chime. It is best that you are running at least Netscape 2.0 or I.E. 3.0. The download site has more information about this plug-in. It is available for Mac, windows (all versions), and some other platforms as well. To download Chime, click below!
Using the Options of the Chime Molecule Viewer
Chime is a powerful 3 dimensional molecule viewer. To
install it, follow the instructions at the download site, or view
its read-me text file. All of its functions are controlled
through a pop-up menu which is accessed by right clicking or hold
clicking anywhere in the image area. You can then alter the type
of model (wire-frame, ball and stick, spacefill, etc.), as well
as color, labeling options, and rotation. If the rotation is
stopped, you can click and hold the molecule with your mouse and
rotate it freely. There is also a stereo function which allows
you to see it in 3 dimensions, if you are talented at crossing
your eyes. It has other features as well, most of which are very
intuitive. See the download site for further information.
Try your hand at Chime right here.
       |
Thanks for visiting!
Back to First
Page (index)