Heroin
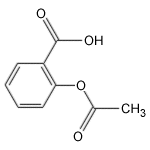
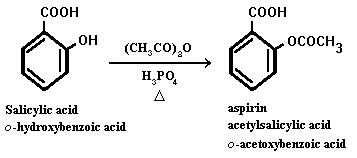
Acetylsalicyclic acid
 How aspirin works
How aspirin works
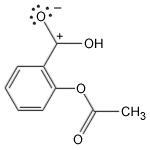
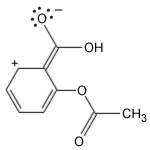
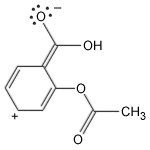
Aspirin, a miracle drug of the 20th century, has changed the lives of millions and has made Bayer an industrial giant. It is a painkiller with minor side effects and has remained unique for decades. The aspirin molecule has an acetyl group linked to a salicyclic acid and is derived from white willow bark. Aspirin begins its work when injuries to human tissues release prostaglandins, hormone-like molecules which cause fever and inflammation. The enzyme responsible for production of prostaglandins is PGHS (prostaglandin H2 synthase), containing two protein subunits, each with a long interior channel. Molecules of arachidonic acid, an essential fatty acid, undergoes a chemical transformation in the enzyme's core. There, it converts to molecules of prostaglandin H2. The aspirin molecule prevents this change with its acetyl group, which binds to a site inside the channel, thus blocking the path of the arachidonic acid. Unfortunately, aspirin shuts down all forms of the PGHS enzyme, including the form that protects the stomach lining. Drug companies are currently developing a new class of painkillers that target only the form of PGHS that causes inflammation.
New uses of aspirin are discovered all the time. While we can take aspirin for safely, we should keep in mind that, like in the case of heroin, science is notorious for modifying facts. It is possible that the medications we take today might reveal harmful effects in the years to come.