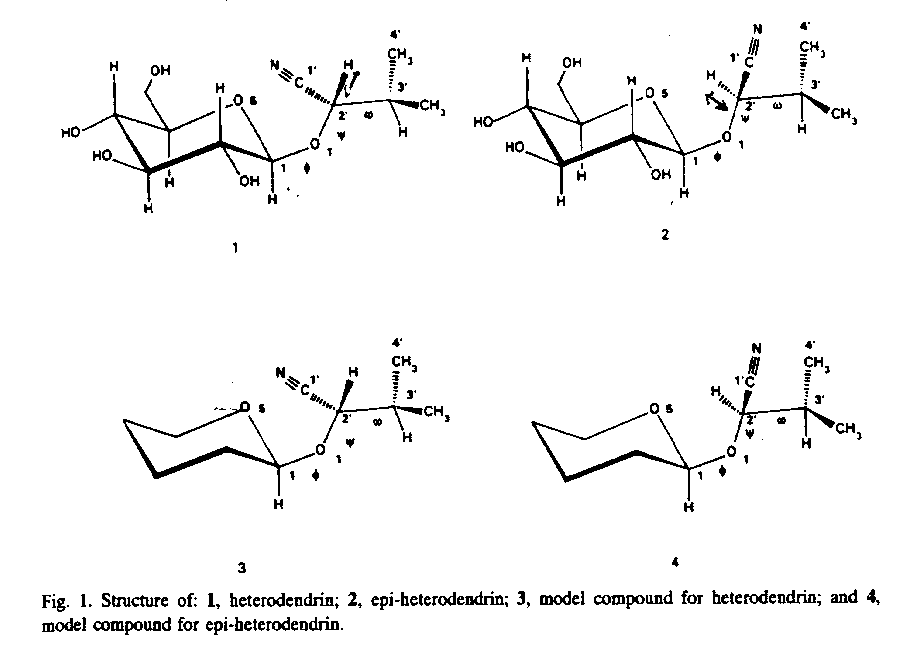
Reference:
Lankhorst, P. P.; Smeets, J. W. H., Haasnoot, C. A. G. Carbohydrate
Research 1995, 269,17-27.
The proton NMR of the two diastereomers differ mainly in the chemical
shift of the H2 in the side chains. However it did not
gave any stereospecific information. Nevertheless the NOE experiment
on a time average basis helped in discriminating between the two
diastereomers.
This was further confirmed by molecular mechanics.

1) Assign each diastereomer to the proton spectra shown.
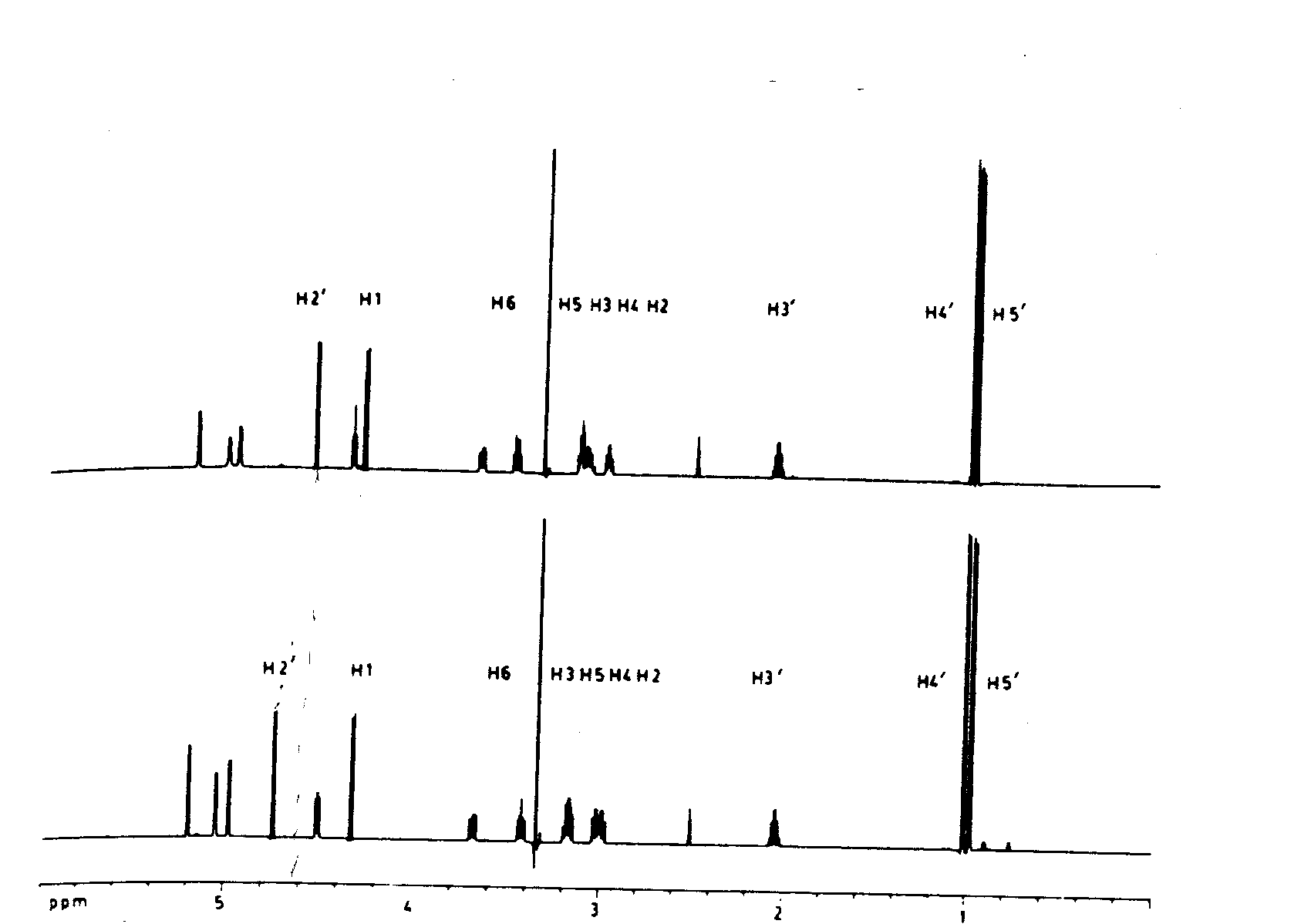
2) Does your assignment of the 1H NMR agree with the structure
and what are the major differences in the 1H NMR of the two diastereomers?
Identification of components and relationships
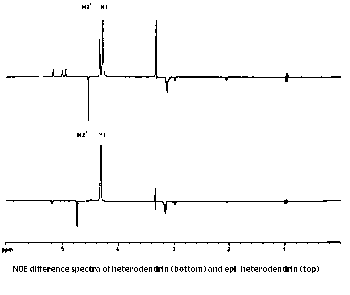
Reasoning using quantitative data
4) Explain the NOED spectra for both the isomers? What information do
you get about the distances between H-1 and H-2' in the two
diastereoisomers?
Support your answer with proper justification.
5) Will the deuteration at 1 and 2' positions in the two
diastereoisomers
affect the NOED?