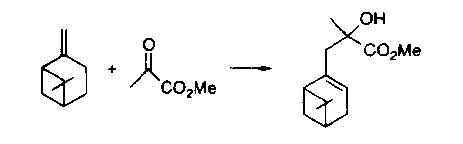 Scheme 1
Scheme 1© 1997, The Fock-ing Computational Chemists are Graeme Day and Mike Lewis. All rights reserved.
|
The Fock-ing Computational Chemists: Graeme Day and Mike Lewis |
In the paper titled Ab Initio Study of the Thio-Ene Reaction. 1. The
Enophile Substituent Effect [Bachrach, S.M.; Jiang, S. J. Org.
Chem. 1997, 62, 8319-8324.], Bachrach and Jiang use high
level correlated ab initio calculations to study the enophile
substituent effect on the transition states and products of various
thio-ene reactions. Calculations were also performed where the enophile
was ethene or formaldehyde. These calculations were necessary to explain
why carbonyls preferentially form alcohols over ethers (Scheme1) in the
ene reaction, while the sulfide is formed preferentially
over the thiol in reactions where a thiocarbonyl acts as the enophile
(Scheme 2). The electronic and steric effects of substituents on
the tiocarbonyl enophile were also considered with regards to the
preference
of sulfides over thiols (Scheme 2).
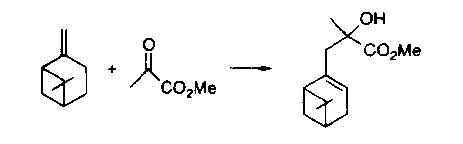 Scheme 1
Scheme 1
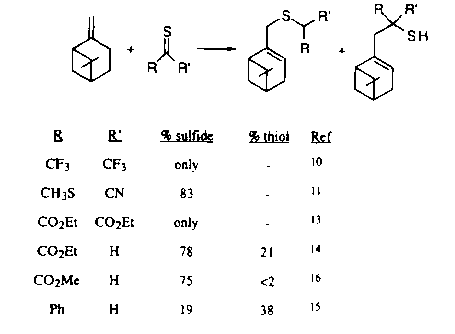
(for references, see paper)
Scheme 2
Previous studies of the related Diels-Alder reaction have shown that the reaction proceeds via a lower activation barrier when thiocarbonyl compounds are used in comparison to Diels-Alder reactions of carbonyls and alkenes. The rationale for these findings is that the C-S pi-bond is weaker than either the C-O or C-C pi-bonds, due to the relatively high HOMOs and low LUMOs in thiocarbonyls. Furthermore, the LUMO is also important in understanding product selectivity (ie. sulfide vs thiol). If the LUMO has greater carbon character, C-C bond formation is prefered and the thiol product is formed, while a greater sulfur character leads to C-S bond formation and the sulfide product. Thus the impetus for this study was to investigate the character and energies of the LUMOs of the enophiles with respect to varying substituents on the thioene.
Stationary points were located and characterized (frequency analysis) at the HF/6-31G* level of theory. The geometries of interest were reoptimized with the inclusion of electron correlation effects using MP2/6-31G* calculations and single-point energies were optained at the MP4/6-31G* level.
Activation and reaction energies of the reactions studied are shown in Table 1. The activation barriers for reactions 2 and 3 confirm that the formation of the alcohol (reaction 2) is favored over the formation of the corresponding ether (reaction 3). Likewise, thiol formation for reactions 4 and 6 have lower activation barriers than the reactions leading to the respective sulfide products (reactions 5 and 7). However, the activation barrier for sulfide formation in reaction 9 is lower (through the exo transition state) than the corresponding thiol formation through reaction 8's favored endo transition state. (For reaction specifics, see paper.)
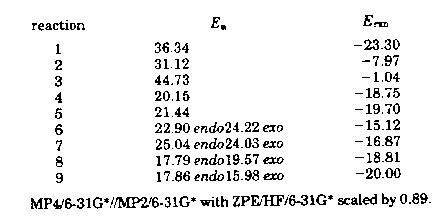
Table 1
The reason for preference of sulfide formation in the reaction pair 8 and 9 is attributed to the effect of the electron withdrawing subtituent on the thioene LUMO. When electron donating substituents are present on the thioene, reaction pairs 4/5 and 6/7, the carbon character of the LUMO is increased, resulting in preferential C-C bond formation and, thus, the thiol. Conversely, electron withdrawing substituents reverse this trend by increaseing the Sulfur character of the LUMO, resulting in C-S formationa and the sulfide. Finally, the activation energies shown in Table 1, can be rationalized by the LUMO energies in Table 2. (NB The HOMO remains constant since the enophile is always reacting with propene.) There is a direct correlation between enophile LUMO energy and activation energy. Thials with electron withdrawing groups have the most stable LUMOs, while those with electron donating groups have less stable LUMOs.

Table 2
QUESTIONS
Q1 Why is the study of transition state geometries, their energies and how they are affected by substituents important? (ICR)
Q2 Based on the findings of this paper, give enophiles which will react with propene to yield a) a thiol, b) a sulfide. Explain your choices of reactants based on arguments used in the paper. (Don't use the same reactants as in the paper. Be original!) (FAR)
Q3 In the reaction SM -> TS4 -> P4, the authors state that this is an early transition state. What geometrical attributes of TS4 allow them to make this statement? (RQD)
Q4 According to FMO theory, what is the significance of the LUMO energy for the enophile? (SCL)
Q5 The authors compare their MP2/6-31G* optimized structures to HF/3-21G optimized structures calculated by Houk et al. Which calculations are mrore reliable and why? (Saying the former level is "higher" will not suffice.) Include discussion on basis sets AND method. (SCL)
GROUP DYNAMICS
The group's first meeting (Feb 4th) was in the departmental reading room, where approximately one hour was spent browsing the Journal of Organic Chemistry, Journal of the Americal Chemical Society and Angewandte Chemie for a recent article.