Electron affinities (EA) were determined experimentally. For COT, EA was obtained from the forward and reverse reaction rate constants for the electron-transfer equilibrium between COT and O2-. The EAs of C8H6 and C8H7 were obtained using photoelectron spectroscopy of C8H6- and C8H7-. The authors used MO calculations to investigate the structures of C8H7- and C8H7. The thermodynamic acidities (DHacid) of C8H9, C8H8, and C8H7 were bracketed by proton-transfer reactions of the corresponding anions. Bond dissociation energies (BDE) for C8Hn (n=7-9) were obtained from EA and DHacid using the following relation:
In addition, BDE of C8H10 were determined by this equation:
| RH | DHacid(kcal/mol) | EA(eV) | BDE(R-H) (kcal/mol) |
| C8H10 | 82.6 +(or-) 3.8 | ||
| C8H9 | -349.9+(or-)4.1 | 49.1+(or-)4.1 | |
| C8H8 | 381.3+(or-)2.3 | 0.55+(or-)0.02 | 93.0+(or-)2.3 |
| C8H7 | 357.2+(or-)8.3 | 1.091+(or-)0.008 | 67.8+(or-)8.3 |
| C8H6 | 1.044+(or-)0.008 |

1. (SCL) Give the definition of adiabatic electron affinity (EA). Why does the large conformational change between the ground states of COT and COT - make the determination of adiabatic EA difficult?
2. (SCL) What reactions form the basis of equation (1) and equation (2)?
3. (RQD) Explain the comparatively low EA value for C8H8 (see Table 1).
4. (RQD) Explain the trend in column 3 of Table 1.
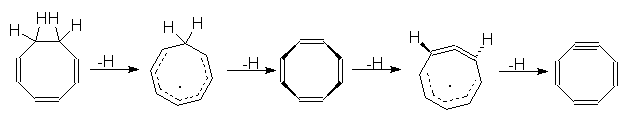
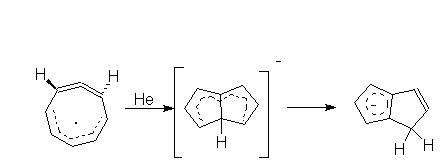
5. (FAR) The rearrangement product of COT - was
predicted
basing on the MO calculation unspecified in the paper. What kind of
calculation
would you do to predict this structure?
The group met 2 times on the first week of February in order to select the paper. First we met in 324 Chemistry to discuss the type of paper required (it took about 15-20 minutes) and then in 115 Chemistry for final selection. We browsed through communications in 1996-97 JACS and Angewante Chemie looking for the papers related to the computational chemistry. We found about a dozen of papers and selected this one. This meeting took about an hour.
Then we met on February 14 in 324 Chemistry to write the problems. We spent about an hour discussing and writing problems. Then Lixin transferred the assignment into an HTML file. We met on February 15 and spent 1.5 hours correcting the mistakes and finishing up the assignment.
As for our experiences, we feel that it was beneficial to do the assignment in the group. Possibility to divide work between partners saved some time. Discussions within the group were also quite helpful both in the identification of the suitable paper and in the creation of problems.
The biggest problem we encountered was to find the suitable paper. Insufficient background in computational chemistry also made life harder.
Our work together didn't go beyond the project. But we find the projects of this kind quite helpful.